Mining enterprises possess abundant copper sulfide ore resources. However, the extraction process, equipment selection, and costs are key considerations for many mining operations. So, how can your recovery rates and grades be improved?
This article will provide a detailed overview of the extraction process flow for copper sulfide ore and guide equipment selection, helping you enhance efficiency and increase economic benefits.

I. Ore Preparation Stage: Crushing and Grinding
Copper sulfide run-of-mine ore generally has a low grade (typically 0.5%-3%), making direct smelting very costly. The first step in extracting copper from copper sulfide ore is crushing and grinding the raw ore to liberate copper mineral particles from the gangue.
1. Ore crushing
First, large pieces of raw copper sulfide ore are subjected to primary crushing using a Jaw Crusher. Then, Impact Crushers or Cone Crushers are used for secondary and tertiary crushing to further reduce the particle size. This process yields copper sulfide ore with a final particle size of less than 10mm.
2. Material Grinding
Fine sand with a particle size of ≤10mm is finely ground to -200 mesh using a Ball Mill. This step enables efficient liberation of copper minerals from the gangue, maximizing metal recovery rates for your project.
3. Coarse Sand Screening
If excessive coarse sand is present, a Classifier can be used for re-screening, with the coarse fraction sent back for grinding, minimizing the occurrence of substandard product.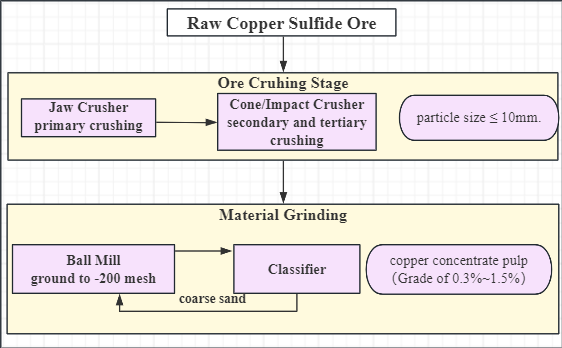
II. Flotation Enrichment – Separating the Copper Ore
The grade of ground copper sulfide ore remains low (0.3% ~ 1.5%), offering minimal profit potential. Enriching the copper ore through chemical and physical methods can increase its grade, creating you greater economic value.
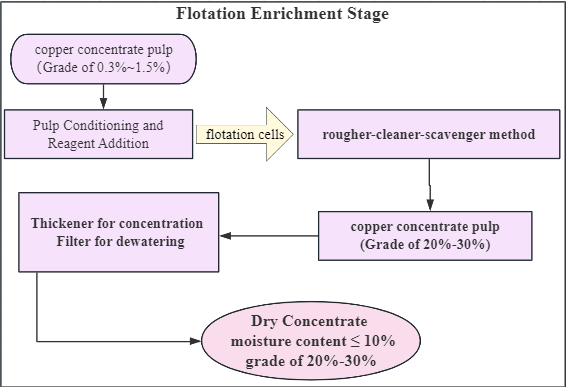
1. Pulp Conditioning and Reagent Addition
Precisely formulate flotation reagents (including frothers, collectors, and modifiers) based on your ore’s characteristics. Optimizing the reagent formula can stably increase the copper concentrate grade to above 20%, thereby enhancing product value and market competitiveness.
2. Aeration and Flotation
After reagent addition, the pulp enters flotation cells or tanks. Copper minerals are separated from the gangue using a rougher-cleaner-scavenger method. Through flotation enrichment, you can obtain copper concentrate pulp with a grade of 20%-30%.
3. Separating Dry Concentrate
Process the copper concentrate pulp: use a Thickener for concentration and a Filter for dewatering. This yields a dry concentrate with a moisture content below 10%, suitable for transportation or further smelting and refining.
III. Pyrometallurgy – High-Grade Purification of Copper Sulfide
Higher grade and purity of copper ore translate to greater value. Pyrometallurgy is currently the most mature industrial method for processing copper sulfide ore and the preferred process for 80% of global copper extraction from sulfide ores. It can upgrade the dry concentrate grade to over 90%.
If your copper sulfide ore grade is ≥20%, and you require high-grade copper extraction on a large scale, choose pyrometallurgy.
It mainly consists of four steps: roasting/drying, smelting, converting, and refining:
Step 1: Roasting/Drying
Dry the copper concentrate to a moisture content ≤0.5%. For high-sulfur copper concentrates, roasting is required first to remove part of the sulfur and harmful impurities like arsenic and antimony, preparing it for subsequent smelting.
Step 2: Smelting
Mix the copper concentrate, flux, and coke in proportion and feed them into a high-temperature furnace (Flash Furnace or Blast Furnace) at 1200-1300°C. This yields matte copper with a grade of 40% ~ 50%.
Step 3: Converting
We perform deep oxidation of the matte copper in a high-temperature converter at 1150-1250°C to remove impurities like iron and sulfur. This process produces blister copper with a grade reaching 98.5%-99.5%.
Step 4: Refining
You can further process the blister copper using fire refining and electrolytic refining. This step is crucial not only for increasing purity but also for securing significant additional profits.
* Fire Refining: Heat the blister copper in a reverberatory furnace for further oxidation and impurity removal, then cast it into anode copper with a grade of 99.5%-99.7%. Anode copper can be sold directly or proceed to the next refining step for higher purity.
* Electrolytic Refining: Subjecting the anode copper to electrolytic refining can produce high-grade cathode copper with purity as high as 99.95% ~ 99.99%, allowing your product to enter high-end markets and command premium prices. Additionally, the anode slime generated during this process is rich in precious metals like gold and silver, providing considerable extra revenue.

IV. Hydrometallurgy – Purifying Low-Grade Copper Sulfide
Although pyrometallurgy is the mainstream technology for processing copper sulfide, some low-grade (0.2%-1%) or refractory ores are more suitable for hydrometallurgy. This process principle involves dissolving copper using chemical reagents, followed by extraction and electrowinning.
Process 1: Sulfation Roaching-Leaching-Electrowinning
Through three steps—roasting conversion, acid leaching purification, and electrowinning—this method can stably convert medium and low-grade sulfide ore into high-purity cathode copper for you, achieving an 85%-90% recovery rate.
Process 2: Bioleaching
This is currently the most environmentally friendly and economically advantageous option. Utilizing microorganisms for natural leaching, it allows you to recover copper resources from ultra-low-grade ores or tailings with 1/10th the energy consumption and 1/5th the investment of pyrometallurgy, with zero sulfur dioxide emissions throughout the process.
Process 3: Pressure Oxidation Leaching – Providing Technical Assurance for Your Challenges
If your ore is high-sulfur and refractory, choose pressure oxidation leaching. It can solve the extraction of finely disseminated, difficult-to-float sulfide ores for you, with recovery rates exceeding 90%.

V. Conclusion
For mining enterprises, optimizing every step in extracting copper from copper sulfide ore can bring tangible benefits. This optimization means you can utilize mineral resources more fully while reducing energy consumption and environmental pollution during production.
Therefore, selecting a suitable extraction process and equipment directly impacts your economic profits. China’s Sandreck Company specializes in selling mining machinery and equipment. Our chief engineers can provide process selection and equipment configuration solutions tailored to your specific needs. If you have any related questions, please feel free to contact us immediately!
FAQs
Q: Why use the “flotation method” to separate minerals instead of smelting the raw ore directly?
A: The flotation method offers a higher return on investment. Direct smelting of copper sulfide ore with a grade <2% incurs excessively high costs and energy consumption. Flotation can enrich the ore to a 20% copper concentrate, which is also more convenient for subsequent smelting and refining.
Q: Does the copper sulfide extraction process cause pollution?
A: Pyrometallurgy for copper sulfide ore emits sulfur dioxide. With current technology, this sulfur dioxide can be captured and converted into sulfuric acid for sale. Process wastewater can also be recycled, creating additional value.
Q: What is the most critical aspect of the grinding?
A: Mineral particle size control. Particle sizes that are too coarse or too fine affect subsequent flotation performance. Complete liberation of copper minerals from the gangue is essential to realize maximum economic value.
Q: Why is lime added during the flotation?
A: Lime primarily acts as a depressant and pulp pH modifier here. It can depress the flotation of impurities like pyrite while creating an alkaline environment suitable for copper mineral flotation.
Q: What is the use of anode slime after electrolysis?
A: It is enriched with precious metals like gold, silver, and platinum, possessing high economic value. These are also significant sources of by-product revenue from copper smelting.





